The C-H bond is one of the most difficult to activate bonds in organic chemistry. The selective activation of inert C-H bonds are of strong interest for the preparation of basic and fine chemicals from natural oil, gas and coal resources and for the synthesis of complex agents, e. g. pharmaceutical products. Nature frequently uses metallo-enzymes for selective C-H activations. The metal ions (mainly Fe and Cu) in the active sites of these enzymes store the oxidation and / or oxygenation equivalents of dioxygen during catalytic cycles. Examples for copper containing enzymes are tyrosinase and particulate methane monooxygenase, while cytochrome P450 and soluble methane monooxygenase are examples for iron containing enzymes for C-H activation.
The active site of the soluble methane monooxygenase (sMMO) contains a diiron core. Upon O2 uptake, it generates a peroxide-bridged diferric intermediate P, which converts to the FeIV(μ-O)2FeIV active species Q, that oxygenates methane to methanol.
In an effort to form structural, electronic, and functional models, we identified amine-phenolate-ligands as strongly electron donating ligands to stabilize high-valent metal ions. The dinuclear FeIII(μ-O)FeIII complex 1 can be reversibly oxidized two times. Spectroscopic and kinetic measurements established a diphenoxyl radical species 12+ that contains the same number of oxidation equivalents as intermediate Q. The dinuclear diphenoxyl radical species decays into mononuclear fragments.
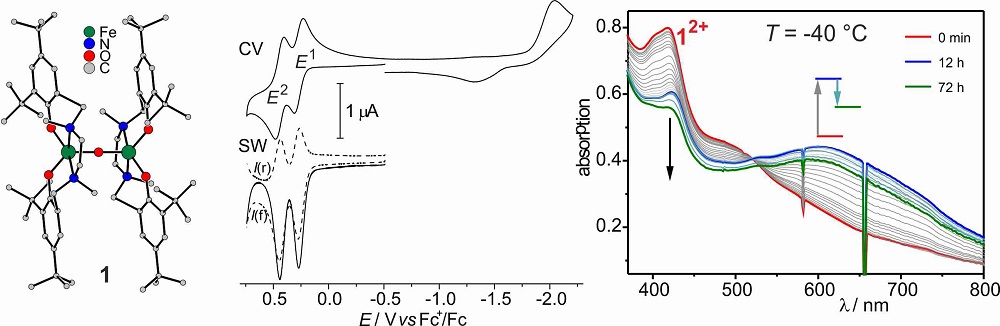
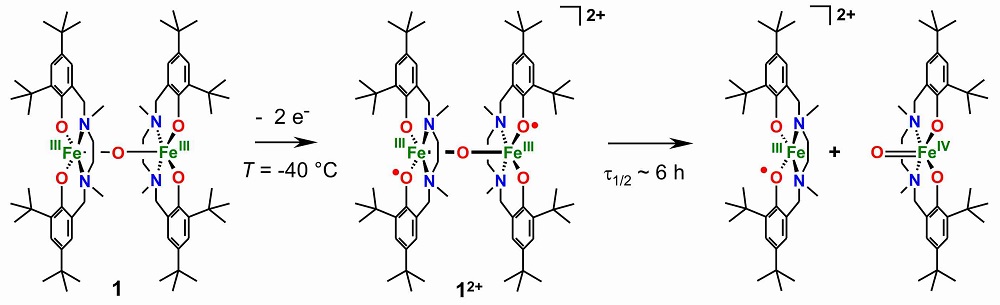
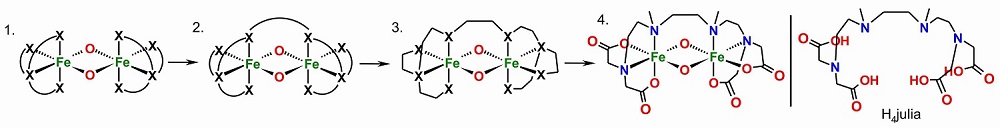
To suppress the dissociation of the oxidized dinuclear complexes and to ensure metal-centered oxidation, we optimized our design for the complexes of the second generation, which resulted in the ligand H4julia.
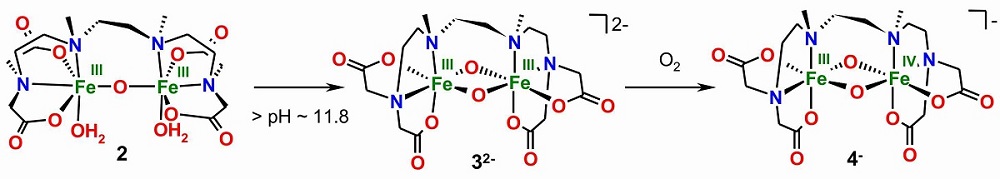
The dinuclear water-soluble complex 2 can be deprotonated twice to 32-. This electron-rich bis-μ-oxo diferric complex 32- can be oxidized in water by O2 to the FeIV(μ-O)2FeIII species 4- with a FeIV h.s. (S=2) ion.
We are currently optimizing the ligand system and vary the metal ions to generate high-valent dinuclear complexes, investigate their electronic structures and reactivities for C-H bond activation.
Related Publications
T. Glaser
"A dinucleating ligand system with varying terminal donor functions but without bridging donor functions: Design, synthesis, and applications for diiron complexes"
Coord. Chem. Rev., 2019, 380, 353-377.T. Zimmermann, S. Dammers, A. Stammler, H. Bögge, and T. Glaser
"Reactivity Differences for the Oxidation of FeIIFeII to FeIII(µ-O)FeIII Complexes Caused by Pyridyl versus 6-Methyl-Pyridyl Ligands"
Eur. J. Inorg. Chem., 2018, DOI: 10.1002/ejic.20180106.M. Aschenbrenner, A. Stammler, H. Bögge, and T. Glaser
"Synthesis and Characterization of a µ-Oxo-Bridged Diferric Complex: An Attempt to Influence the Conformation by Changing the Spacer"
Z. Anorg. Allg. Chem, 2018, 644, 1439-1444.T. P. Zimmermann, T. Limpke, A. Stammler, H. Bögge, S. Walleck, and T. Glaser
"Variation of the Molecular and Electronic Structures of µ-oxo Diferric Complexes with the Bridging Motive"
Z. Anorg. Allg. Chem., 2018, 644, 683-691.T. P. Zimmermann, T. Limpke, N. Orth, A. Franke, A. Stammler, H. Bögge, S. Walleck, I. Ivanovic-Burmazovic, and T. Glaser
"Two Unsupported Termial Hydroxido Ligands in a µ-oxo Ferric Dimer: Protonation and Kinetic Lability Studies"
Inorg. Chem, 2018, 57, 10457-10468.T. P. Zimmermann, T. Limpke, A. Stammler, H. Bögge, S. Walleck, and T. Glaser
"Reversible Carboxylate Shift in a µ-oxo Diferric Complex in Solution by Acid-/Base-Addition"
Inorg. Chem, 2018, 57, 5400-5405.T. Limpke, C. Butenuth, A. Stammler, H. Bögge and T. Glaser
"A Series of Copper Complexes of a Dinucleating Bis(tetradentate) Nitrogen Ligand: Synthesis, Structural, Spectroscopic, Electrochemical, and Magnetic Characterization"
Eur. J. Inorg. Chem, 2017, 29, 3570-3579.S. Dammers, T. P. Zimmermann, S. Walleck, A. Stammler, H. Bögge, E. Bill, and T. Glaser
"A Mixed-Valence Fluorido-Bridged FeII-FeIII Complex"
Inorg. Chem, 2017, 56, 1779-1782.J. B. H. Strautmann, S. Dammers, T. Limpke, J. Parthier, T. P. Zimmermann, S. Walleck, G. Heinze-Brückner, A. Stammler, H. Bögge, and T. Glaser
"Design and Synthesis of a Dinucleating Ligand System with Varying Terminal Donor Functions that Provides no Bridging Donor and its Application to the Synthesis of a Series of FeIII-μ-O-FeIII Complexes"
Dalton Trans., 2016, 45, 3340-3361.G. Heinze-Brückner, S. Walleck, J. B. H. Strautmann, A. Stammler, H. Bögge, and T. Glaser
"Synthesis and Characterization of a Dinuclear Ferric Complex with Redox-active Ligands" "
Inorg. Chim. Acta (Wolfgang Kaim Honorary Issue), 2011, 374, 385-391.J. B. H. Strautmann, S. Walleck, H. Bögge, A. Stammler, and T. Glaser
"A Taylor-made Ligand to Mimic the Active Site of Diiron Enzymes: An Air-Oxidized High-Valent FeIII h.s.(µ-O)2FeIV h.s. Species"
Chem. Commun. 2011, 47, 695-697.J. B. H. Strautmann, C.-G. v. Richthofen, S. DeBeer, E. Bothe, E. Bill, T. Weyhermüller, A. Stammler, H. Bögge, and T. Glaser
"Molecular and Electronic Structures of Dinuclear Iron Complexes Incorporating Strongly Electron-donating Ligands: Implications for the Generation of the One- and Two-Electron Oxidized Forms"
Inorg. Chem. 2011, 50, 155-171.J. B. H. Strautmann, C.-G. Frhr. von Richthofen, S. DeBeer George, E. Bothe, E. Bill, and T. Glaser
"Highly Oxidized Diiron Complexes: Generation, Spectroscopy, and Stabilities"
Chosen as Chem. Comm. Hot Article
Chem. Comm. 2009, 2637-2639.J. B. H. Strautmann, S. DeBeer George, E. Bothe, E. Bill, T. Weyhermüller, A. Stammler, H. Bögge, and T. Glaser
""Molecular and Electronic Structures of Mononuclear Iron Complexes and their Oxidizing Forms Using Strongly Electron-donating Ligands""
Inorg. Chem. 2008, 47, 6804-6824.T. Glaser, R. Pawelke, and M. Heidemeier
"Synthesis, Structure, and Spectroscopic Properties of a Dinuclear Fe(III)-(µ2-O)-Fe(III) Complex Using a Strongly Electron-Donating Ligand: Implications for the Generation of New High-Valent Species"
Z. Anorg. Allg. Chem. (Bernt Krebs Honorary Issue) 2003, 629, 2274-2281.